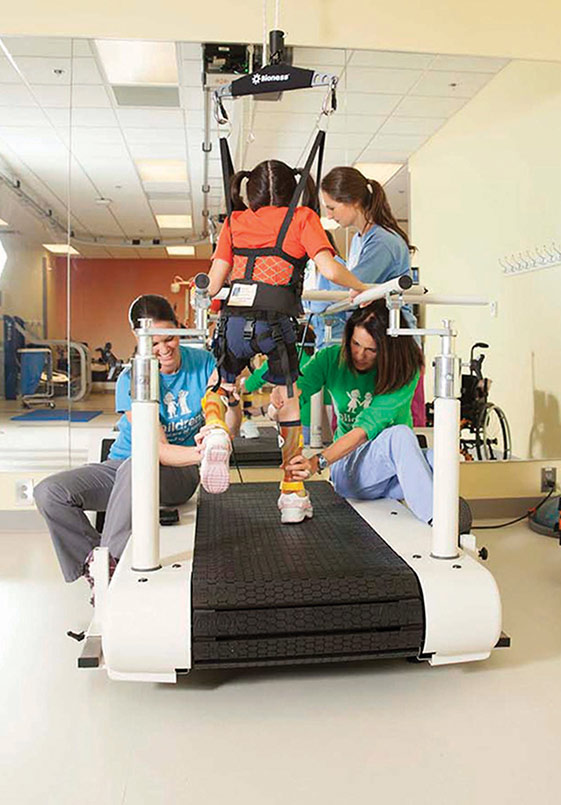
Cerebral palsy (CP) can decrease mobility, which is key to quality living. Children with CP and compromised mobility are at risk for low bone mineral density and fragility fractures, but physical therapy programs and orthoses can help kids be more active to build stronger bones.
By Hank Black
Normal childhood development of a strong skeleton, which is dense in minerals such as calcium and required for optimal mobility, depends partly on the ability to load weight through the long bones of the legs. Children who may not be able to stand independently or ambulate, including many with CP, are at risk of having low bone mineral density (LBMD) and fragile bones, comorbidities that can have long-term consequences. Inactive younger adults are at risk of increased morbidities that adversely affect the quality of their lives, and sedentary older adults are prone to earlier death.
CP is the most common pediatric physical disability and affects 3.6/1000 of children.1 Their caregivers and practitioners have a number of ways to cope with LBMD, including supported standing programs and weightbearing activities, as well as lower extremity orthoses to enable those interventions. More recently, medical therapies previously used primarily in adults have become an option in the pediatric population.
Major gaps in research, however, bedevil the field, partly due to adult and pediatric funding disparities. Dino Scanio, CO, LO, of Pediatric Orthotics and Prosthetics Services of Tampa, FL, who works with many children with CP, said orthotics research is one area of concern. “It’s more common that orthotic research advances are mainly in the adult arena. There needs to be equal effort in helping children with special needs. We have seen advances in the last several years, but most significantly in prosthetics, while orthotics falls behind,” he said.
Parents of children with CP at risk of having LBMD eagerly await the availability of technological advances such as whole body vibration (WBV), exoskeletons, and newer orthotic materials. New research findings, however, are sometimes slow to filter down to parents.
Jennifer Lyman of New Orleans, LA, cares for her son Bower, aged 11 years, who is classified as having moderate to severe CP (levels IV to V on the five-step classification of the Gross Motor Function Classification System [GMFCS]).2 Lyman said, “I help coordinate a collaborative of national cerebral palsy organizations but had never heard of whole body vibration until just last year.”
Understanding fracture risk

The Center for Advanced Technology and Robotic Rehabilitation uses many modalities to improve mobility in kids with CP and other disorders. (Photo courtesy of Children’s Healthcare of Atlanta.)
Bones with LBMD are prone to fracture even in the absence of discernible trauma.3 Over their lifetime, children with higher levels of movement disorders such as CP show a diminishing rate of mineralization compared with that of healthy children.4 Nonambulatory children and young adults with CP have close to a 20% prevalence rate for fracture, and a 4% annual fracture incidence.3 Most commonly, lower extremity fractures involve the distal femur.5
Approximately 80% of patients with severe CP have LBMD,3 and the overall incidence rate of fracture in that classification is between 7% and 9.7%.6 Wort et al7 in a 2013, nine-year epidemiological retrospective study of 536 children in Sweden, found higher functioning children (GMFCS levels I to III) had a similar incidence of fractures as children with normal development. Among children in levels IV to V (28%), however, risk of nontraumatic fracture was significantly increased in those who did not use standing devices (18%) and had stunted growth (height for age < -5 SD; 31%), which the investigators attributed largely to suspected nutritional deficiency. In addition, participants who took antiepileptic drugs (64%) had twice the risk of fracture, and children in GMFCS levels IV to V who had a gastrostomy (typically performed for dysphagia or nutritional support) also had an increased risk of nontraumatic fractures.
Typically, density of bones is measured by dual x-ray absorptiometry (DXA) and results expressed as z scores calculated from age, gender, and ethnicity-adjusted norms.2 Children are diagnosed with fragile osteoporosis in the presence of LBMD (z scores < -2) together with a history of a fracture of the long bones of the legs, a vertebral compression fracture, or multiple fractures of the upper extremities.3
Conducting DXA on children with CP can be problematic due to contractures and other intrinsic problems, so scans often are not prescribed until a bone is broken. Emerging diagnostic technologies such as 3D quantitative computed tomography and, more recently, quantitative ultrasound offer additional information, but their use is limited by higher radiation exposure and lack of standardization, respectively.8
Weightbearing programs
Pediatric supported-standing programs are in widespread clinical use. There is some evidence that such programs increase BMD but not that they decrease fragility fractures. In a 2011 review, Fehling et al found inadequate data to support a recommendation for weightbearing activities to improve BMD or for decreasing fragility fractures in children with CP.9 In a 2010 review, Hough et al10 found a significant increase in BMD of the lumbar spine but not the proximal tibia for one small trial of static standing involving nonambulatory patients.11 A 2010 review by Glickman et al included 10 studies of children with neuromuscular disorders including CP and reported moderately strong evidence that supported-standing programs increased BMD in the legs and spine, and also some evidence that it decreased spasticity and improved range of motion.12
 In the absence of negative side effects of these activities, practitioners and caretakers have been using them for the possible BMD benefit as well as for stretching, range of motion maintenance, pressure relief, mental clarity, and because children often enjoy the activity.9,13
In the absence of negative side effects of these activities, practitioners and caretakers have been using them for the possible BMD benefit as well as for stretching, range of motion maintenance, pressure relief, mental clarity, and because children often enjoy the activity.9,13
The optimal dose for supported standing to positively affect BMD in pediatric patients is 60 to 90 minutes per day for five days a week, according to Ginny S. Paleg, PT, DScPT, of Silver Spring, MD, who led a 2013 review on the topic. Based on evidence from 30 studies as well as the authors’ opinions, the review also recommended dosing standards for hip stability (60 mins/d); range of motion for hip, knee, and ankle (45 to 60 mins/d); and spasticity (30 to 45 mins/d).13
Some studies analyzed in the review showed standing programs can be safely started as early as nine to 10 months of age, Paleg said. Lyman, the New Orleans parent, said, “There’s really no way to tell why my son seems to have strong bones with no fractures to this point, but when he was 18 months old, he started using a jumper seat that hangs from a doorway and allowed him to put weight through his legs.” A year later, Bower was using a gait trainer, an assistive tricycle, and other devices for weightbearing and muscle development. She also has provided him with good nutrition and vitamin D supplementation for bone health.
Paleg, a pediatric and early intervention physical therapist, said, “Interventions should be multifactorial. We find standing or exercise programs by themselves help in many ways, and should be accompanied by good nutrition, sunlight, vitamin D and calcium supplementation, and medications as needed. I am most excited about combining the emerging technology of whole body vibration with activity programs that include concepts of explorative play [that are new to the US] as developed by Swedish physical therapist Ylva Dalén some 10 years ago.”
WBV training uses oscillatory motion around an equilibrium point and is used as a coadjuvant modality to improve motor function and functional performance.14 It’s practiced on a vibrating standing platform in a static or dynamic position.15 Saquetto et al in a 2015 meta-analysis of six randomized controlled studies found WBV may improve BMD, gait speed, and standing function in children with CP, but could not make detailed recommendations due to small study size and variability in protocols and patient characteristics.14
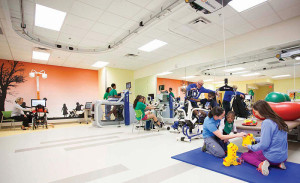
Kids at the Atlanta center work on improving motor function and functional performance. (Photo courtesy of Children’s Healthcare of Atlanta.)
Therapists at Children’s Healthcare of Atlanta’s Center for Advanced Technology and Robotic Rehabilitation have used WBV with children with CP and other disabilities since 2014 and have treated about 40 children with CP of different levels, according to physical therapist Erin Eggebrecht, PT, DPT, NCS.
“Our kids call the vibration device the ‘milkshake machine,’ and we’re glad to have it as part of our overall program,” she said. “If the children have a lot of spasticity the device helps them relax, but if they are hypotonic, it allows them to stand up longer. There seem to be good immediate effects as a warm up to functional skill training such as grasp-and-release for upper extremities. For lower extremities the effect may not be as dramatic, but it is still beneficial. Our ambulatory patients have experienced gains in holding a single-leg stance immediately after WBV, as well as improved upright positioning with walking.”
Eggebrecht said, “We use WBV at the start of a session for up to 15 minutes and find it increases proprioception and balance, setting the stage for following activities with greater sensory awareness. Kids might say their legs itch or feel warm, and the older ones might say they feel lighter when they get off of it. Some take a while to get used to the feeling, but ultimately most of them love the input they receive.”
She expressed hope that research will provide more concrete information on the therapy’s effect on BMD as well as determining how long effects continue. “This device has the potential to help low BMD, while being easy to set up and operate in a safe environment. We also hope to see our kids return and report changes outside of therapy from using WBV,” she said.
Advances in orthoses
Standing frames and weightbearing therapeutic activities should be used daily for nonambulatory children, according to Curt Bertram, CPO, who practices in Hartland, WI, and is a director of Austin, TX-based Hanger Clinic’s National Residency Program. In addition to weightbearing for children with GMFCS levels I to III, mobility is important in maintaining good health. Mobility-enhancing concepts in ankle foot orthosis (AFO) alignment and materials such as silicone are entering today’s pediatric orthotics practice, he said.
“AFO tuning, as it relates to lower extremity AFO alignment, is a hot topic in pediatric orthoses,” Bertram said. Based on research from the UK, tuning puts the gait cycle and the ground reaction vectors in the appropriate alignment with the knee and hip so training can be focused on unrestricted parts of the body over which the patient has better control.16,17
Mark K. Taylor, MLS, CPO, of the University of Michigan Orthotics and Prosthetics Center in Ann Arbor, agreed tuning allows for better fitting of AFOs. “Proper hind- and midfoot alignment requires the appropriate degree of range of motion, and the orthotist and physical therapist working together make a great team to ensure the best fit,” he said.
Taylor said weight gain is a major problem in CP as children in GMFCS level III become less active with age and stop ambulating. “Weight is the number one challenge for independence and a big issue in mobility. Obese kids have to work so much harder to get up and be mobile,” he said. Composite materials such as carbon fibers can make orthoses stronger, and better knowledge of forces that can control the rotational plane of traditional plastics help practitioners produce more effective AFOs, Taylor said.
Carbon fiber and other energy-storing materials used in dynamic bracing are now being introduced for pediatric orthoses and will help with mobility, Taylor added. “It’s neat stuff, but we’ve got to learn how to work with it properly. We’ve used it in prosthetics for years because we could put it anywhere inside. In orthoses, we’ve had to create designs that put material on the outside of the lower extremity.”
Bisphosphonates
Bisphosphonates are used to improve LBMD by inhibiting bone resorption. They are used in adults to reduce bone loss and particularly to prevent fragility fractures in the elderly. Over the past decade, bisphosphonate use has expanded to include certain pediatric patients, including those with CP.
Numerous clinical centers are using pamidronate, zoledronate, and occasionally other drugs to reduce fracture risk in these vulnerable populations, according to Laura L. Tosi, MD, an orthopedic surgeon at Children’s National Health System in Washington, DC.
Reports on bisphosphonate use in children with osteogenesis imperfecta,18 Duchenne muscular dystrophy,19 and CP20 underscore this trend in clinical practice. An early report from a small controlled clinical trial demonstrated that pamidronate was a safe and effective agent for increasing BMD in nonambulatory children with CP.3
Perhaps most exciting, Julieanne P. Sees, DO, and colleagues at Nemour/Alfred I DuPont Hospital for Children in Wilmington, DE, in a recent retrospective review of 32 children with CP (GMFCS levels III-V), looked beyond BMD and found that pamidronate treatment was associated with a highly statistically significant reduction in fracture rate in those patients with an average follow-up of six years or longer.21
Sees said the annual rate of fracture dropped from 2.4 per year pretreatment to .10 per year post-treatment. Of the children studied, 27 were GMFCS level V patients. “We and the parents were very pleased at the results, which give them some relief from stress over the low-energy injuries that usually had no obvious mechanism of injury,” she said.
Tosi is coauthor of a 2013 review of treatment with bisphosphonates for children with various disabilities.22 “As there are still no clear guidelines for children, we generally limit our treatment of children with CP to those who have had two or more fractures,” she said.
“Our Achilles heel is the absence of randomized controlled trials that would provide definitive guidance,” Tosi said. She is excited that George et al23 and Otaify et al24 have provided first steps in defining standardized dosages, intervals, procedures, and anticipated adverse effects in the use of bisphosphonates among children with skeletal dysplasia.
Hank Black is a medical writer based in Birmingham, AL.
- Yeargin-Allsopp M, Van Naarden Braun K, et al. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics 2008;121(3):547-54.
- International Society for Clinical Densitometry (ISCD). Pediatric official positions of the ISCD, West Hartford, CT, 2007. J Clin Densitom: Assessment of Skeletal Health, 2008;11(1):6e
- Henderson RC, Lark RK, Gurka M, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics 2002;110:e5.
- Henderson RC, Kairalla JA, Barrington JW, et al. Longitudinal changes in bone density in children and adolescents with moderate to evere cerebral palsy. J Pediatr 2005;146:769-775.
- Munns CFJ, Cowell CT. Prevention and treatment of osteoporosis in chronically ill children. J Musculoskelet Neuronal Interact 2005;5(3):262-272.
- Stevenson RD, Conaway M, Barrington JW, et al. Fracture rate in children with cerebral palsy. Pediatr Rehabil 2006;9(4):396-402.
- Wort UU, Nordmark E, Wagner P, et al. Fractures in children with cerebral palsy: a total population study. Dev Med Child Neurol 2013;55:821-6.
- Boyce AM, Tosi LL, Paul SM. Bisphosphonate treatment for children with disabling conditions. Phys Med Rehabil 2014 May;6(5):427-36.
- Fehlings D, Switzer L, Agarwal P, et al. Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk for osteoporosis: a systematic review. Dev Med Child Neurol 2012;54:106-116.
- Hough JP, Boyd RN, Keating JL. Systematic review of interventions for low bone mineral density in children with cerebral palsy. Pediatrics 2010;125:e670-8.
- Caulton JM, Ward KA, Alsop CW, et al. A randomized controlled trial of standing program on bone mineral density in nonambulant children with cerebral palsy. Arch Dis Child 2004;89(2):131-135.
- Glickman LB, Geigle PR, Paleg GS. A systematic review of supported standing programs. J Pediatr Rehab Med 2010;3(3):197-213.
- Paleg GS, Smith BA, Glickman LB. Systemic Review and evidence-based clinical recommendations for dosing of pediatric supported standing programs. Pediatr Phys Ther 2013;25(3):232-247.
- Saquetto M, Carvalho V, Silva C, et al. The effects of whole body vibration on mobility and balance in children with cerebral palsy: a systemic review with meta-analysis. J Musculoskelet Neuronal Interact 2015;15(2):137-144.
- Ruck J, Chabot G, Rauch F. Vibration treatment in cerebral palsy: a randomized controlled pilot study. J Musculoskelet Neuronal Interact 2010;10(1):77-83.
- Fatone S, Stine R, McGovern D, Pavone L. Improving lower extremity orthotic management of children with cerebral palsy: AFO-FC case studies. Presented at American Academy of Orthotists and Prosthetists Annual Meeting & Scientific Symposium, Chicago, February 2014.
- Jagadamma KC, Owen E, Coutts FJ, et al. The effects of tuning an ankle foot orthosis footwear combination on kinematics and kinetics of the knee joint of an adult with hemiplegia. Prosthet Orthot Int 2010;34(3):270-276.
- Palomo T, Fassi F, Ouellet J, et al. Intravenous bisphosphonate therapy of young children with osteogenesis imperfecta: skeletal findings during follow up throughout the growing years. J Bone Min Res 2015;30(12):2150-2157.
- Sbrocchi AM, Rauch F, Jacob P, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporosis Int 2012;23(11):2703-2711.
- Kim MJ, Kim SN, Lee IS, et al. Effects of bisphosphonates to treat osteoporosis in children with cerebral palsy: a meta-analysis. J Pediatr Endocrinol Metab 2015;28(11-12):1343-1350.
- Sees JP, Sitoula P, Dabney K, et al. Pamidronate treatment to prevent reoccurring fractures in children with cerebral palsy. J Pediatr Orthop 2015 Mar 6 [Epub ahead of print]
- Boyce AM, Tosi LL, Paul SM. Bisphosphonate treatment for children with disabling conditions. Phys Med Rehabil 2014;6(5):427-436.
- George S, Weber DR, Kaplan P, et al. Short-term safety of zoledronic acid in young patients with bone disorders: an extensive institutional Experience. J Clin Endrocrinol Metab 2015;100(11):4163-4171.
- Otaify GA, Aglan MS, Ibrahim MM, et al. Zoledronic acid in children with osteogenesis imperfecta and Bruck syndrome: a 2-year prospective observational study. Osteoporos Int 2016;27(1):81-92.